DIN 12980 Cytotoxic Safety Cabinets – TÜV Nord Certified for Maximum Protection
Labman Instruments is official distributor for CleanAir by Baker Biovanguard B cytotoxic safety cabinets and other laboratory hoods for Belgium.

Advanced DIN 12980 Compliant Class II BioVanguard B Cabinets for Safe Handling of Cytotoxic and High‑Risk Materials
Our Biovanguard B Series cytotoxic safety cabinets are TÜV Nord certified according to both EN 12469 and DIN 12980. Designed specifically for the safe handling of hazardous drugs and cytostatics in GMP-compliant environments, these Class II cabinets are ideal for hospital pharmacies, oncology labs, and cleanrooms. With industry-leading safety features and the lowest cost of ownership in the market, the Biovanguard B Series ensures total protection for operator, product, and environment.
Unlike standard biological safety cabinets, they are designed to safely manage contaminated filters without releasing toxic particles into the air—ensuring full compliance with DIN 12980 and keeping your staff and environment safe.
The CleanAir by Baker Biovanguard B series is certified by TÜV Nord to the EN 12469 and DIN 12980 guidelines, ensuring operator safety when working with cytotoxic drugs or other hazardous products. The perfect biosafety cabinet solution for chemotherapy preparations in hospital pharmacies or research centra.
Biovanguard B with Manual Front Window – Maximum Safety and Full Control for GMP Workflows
The Biovanguard B Series with manual sash is the ideal cytotoxic safety cabinet for pharmaceutical laboratories and hospital pharmacies seeking maximum protection with straightforward operation. Fully TÜV Nord certified according to EN 12469 and DIN 12980, this Class II cabinet ensures user, product, and environmental protection during the handling of hazardous drugs.
Designed with energy efficiency, long filter life, and low noise levels, the Biovanguard B offers the lowest cost of ownership in its class. Standard features include a large ergonomic work surface, audible and visual alarms, and H14 HEPA filtration to meet the strictest GMP and PIC/S Annex 3 standards.

- Cytotoxic cabinet
- Internal width: 1180 mm
- Compliance: TÜV Nord certified to EN12469 & DIN 12980
- Motors: 3 x energy efficient EC motors
- Sash type: manual sliding sash
Price on request

- Cytotoxic cabinet
- Internal width: 1790 mm
- Compliance: TÜV Nord certified to EN12469 & DIN 12980
- Motors: 5 x energy efficient EC motors
- Sash type: manual sliding sash
Price on request
Biovanguard B with Electric Front Window – Effortless Operation and Premium GMP Protection
The Biovanguard B Series with electric front sash combines the highest level of safety with unmatched ease of use. Ideal for GMP-compliant cleanrooms and pharmacy compounding environments, the motorized window allows seamless, hands-free operation at the push of a button.
Certified by TÜV Nord according to EN 12469 and DIN 12980, this cabinet is purpose-built for the safe manipulation of cytostatics and other hazardous drugs. It supports full IQ/OQ qualification, features automatic airflow control, energy-saving modes, and delivers exceptionally low operational costs thanks to smart design and minimal maintenance needs.

- Cytotoxic cabinet
- Internal width: 1180 mm
- Compliance: TÜV Nord certified to EN12469 & DIN 12980
- Motors: 3 x energy efficient EC motors
- Sash type: electrical sliding sash
Price on request

- Cytotoxic cabinet
- Internal width: 1790 mm
- Compliance: TÜV Nord certified to EN12469 & DIN 12980
- Motors: 5 x energy efficient EC motors
- Sash type: electrical sliding sash
Price on request
What is DIN 12980 and Why It Matters for Cytotoxic Safety Cabinets
DIN 12980 is the key European standard that defines the design, safety, and performance requirements for cytotoxic safety cabinets. Unlike general biosafety cabinets covered under EN 12469, DIN 12980 includes stricter criteria specifically for the manipulation of hazardous drugs, such as:
- Higher minimum inflow velocity (≥ 0.40 m/s)
- Triple HEPA filtration for maximum containment
- Safe filter replacement systems to prevent exposure
- Validated design for safe handling of cytostatics and chemotherapy agents
Our Biovanguard B cabinets are independently tested and certified by TÜV Nord to meet all DIN 12980 requirements. This guarantees compliance with EU GMP Annex 1 and PIC/S Annex 3 guidelines, making them the ideal solution for hospital pharmacies and pharmaceutical cleanrooms handling cytotoxic substances.
Frequently asked questions about DIN 12980 Safety Cabinets
What is DIN 12980?
DIN 12980 is a European standard that defines the safety requirements for safety cabinets used in the preparation of cytotoxic drugs. It goes beyond EN 12469 with additional criteria for inflow velocity, filtration, and containment safety.
Why choose a DIN 12980-certified cabinet for cytostatics?
DIN 12980-certified cabinets offer triple-filter protection, higher inflow speed, and safer filter replacement, making them essential for safe cytostatic drug handling in hospital and pharma labs.
What is a cytotoxic safety cabinet and how does it differ from a standard biosafety cabinet?
Why Standard Biological Safety Cabinets Are Not Always Enough for Cytotoxic Drug Handling
Biological safety cabinets (BSCs) are engineered to protect both the user and the surrounding environment from exposure to biohazards, while maintaining a sterile ISO Class 5 work area. To achieve this, they rely on HEPA filtration, which efficiently captures airborne micro-organisms and particles.
While HEPA filters also trap hazardous powders—including cytotoxic and antineoplastic drugs—standard BSCs are not designed for the safe handling of these compounds during filter maintenance. Here’s why:
When a biological safety cabinet is used for cytotoxic products, the HEPA filters become contaminated with hazardous drug residues. Unlike biological agents, cytotoxic compounds cannot be neutralized by gaseous decontamination (e.g., formaldehyde or hydrogen peroxide vapor). As a result, there is a significant risk that these toxic substances become airborne again during filter replacement, posing a serious occupational exposure risk.
Why You Need a Dedicated Cytotoxic Safety Cabinet
Cytotoxic safety cabinets, also referred to as DIN 12980-compliant cabinets, are specifically designed to mitigate this risk.
They include a third HEPA filter positioned beneath the work surface, which serves as an additional containment layer.
This pre-exhaust filter can be safely replaced while the cabinet is running, ensuring continuous negative pressure to prevent release of contaminants.
The extra filtration step significantly reduces the risk of cytotoxic agents escaping into the environment during maintenance or filter change.
Key Benefits of Cytotoxic Cabinets for Hazardous Drug Compounding
Enhanced protection for personnel, product, and environment
Safe filter replacement under continuous negative pressure
Compliant with DIN 12980 and other international standards for cytotoxic drug handling
Suitable for hospital pharmacies, oncology preparation units, and pharmaceutical manufacturing environments
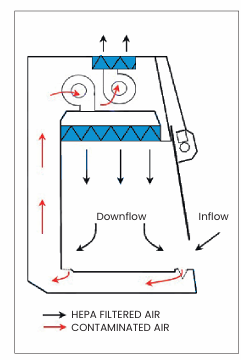
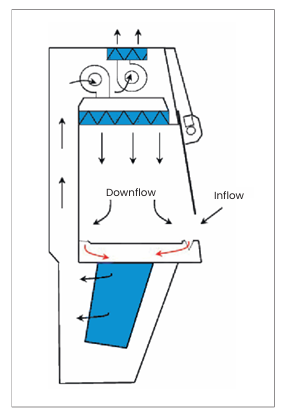
Does my cytotoxic cabinet need to be ducted?
If you’re working with cytotoxic or antineoplastic drugs in a regulated environment, a ducted cytotoxic safety cabinet is the safest and most compliant choice. Even when HEPA filters are in place, ducting ensures residual vapors or accidental leaks don’t recirculate into the workspace.
If the cytotoxic safety cabinet is used for sterile compounding of other hazardous products, please check the safety datasheets for requirements about ducting.
Can cytotoxic cabinets be used for sterile compounding?
Yes, cytotoxic cabinets are suitable for sterile preparations, including those requiring aseptic techniques, as long as the cabinet is installed in a controlled cleanroom and regularly validated for ISO 5 performance.
Biovanguard B cytotoxic cabinets offer the possibility to be delivered with 0,45m/s downflow speed to easily comply with GMP Annex 1 or PICS annex 3.
Are your cytotoxic cabinets compliant with GMP and DIN 12980 standards?
Our Biovanguard B cytotoxic cabinets comply with all safety standards:
- TÜV NORD certified to the EN 12469 standard for biological safety cabinet
- TÜV NORD certified to the DIN 12980 standard for laboratory installations – Safety cabinets and glove boxes for cytotoxic substances and other CMR drugs
TÜV NORD CERT offers manufacturers and users support through product inspections in accordance with the requirements of the Product Safety Act. Our experts, whose decades of experience have significantly shaped the relevant product standards, help you establish and maintain a high level of safety.
The GS mark stands for quality and safety in Europe and many other countries worldwide. It indicates that the product, including its instructions for use, has been tested by an independent institution. Products with the GS mark are favored due to their proven safety and offer a clear competitive advantage.
On top of external EN 12469 and DIN 12980 certifications, our Biovanguard B cytotoxic cabinets can also be configured with 0,45 m/s downflow speed to comply with GMP guidelines.
What biosafety cabinet is used for chemotherapy?
For chemotherapy drug preparation, a Class II Type II A2 biological safety cabinet specifically designed for cytotoxic applications is used—commonly referred to as a cytotoxic safety cabinet. These cabinets, like the Baker BioVanguard B, offer triple protection: for the product, the operator, and the environment. They comply with standards such as DIN 12980, EN 12469, and GMP Annex 1, and are ideal for handling hazardous drugs in hospital pharmacies, especially during oncology and chemotherapy compounding.
Can you provide IQ/OQ validation for DIN 12980 cabinets?
Absolutely. We offer full IQ/OQ qualification services on-site in Belgium, in line with GMP and PIC/S Annex 3 requirements.
Who should use a DIN 12980 cabinet?
These cabinets are essential in hospital pharmacies, oncology departments, pharmaceutical cleanrooms, and any GMP environment handling cytotoxic or hazardous drugs.
Get Expert Advice on DIN 12980-Compliant Safety Cabinets
Contact us today for a quote, technical documentation, or on-site qualification services for your hospital or GMP-certified laboratory.

